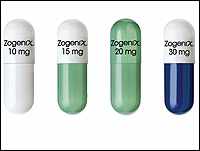
Controversial approval of new prescription drug Zohydro stirs passions on both sides
FDA approval of Zohydro faces post-approval criticism
Prescription drug abuse and misuse occurs across the globe, and the recent FDA approval of a new medication, Zohydro, has stirred passions on both sides.
Overall, chronic pain patients are pleased with the approval.
Zohydro, a pure form of hydrocodone, does not have any secondary ingredients which means that patients can be prescribed the drug in adequate amounts to control pain.
Other similar painkillers on the market, such as Vicodin, have either acetaminophen or ibuprofen added to the formulation. The reason behind the additives is to purportedly control abuse, since both additives can cause irreversible damage to the patient if taken in large quantities, however, it also limits how much pain medication a patient can take, and that decision seems to have been made to protect street drug abusers rather than find a better quality of life for medical patients.
Unfortunately, the prescribed medications which can provide immeasurable pain relief to chronic pain patients and improve their quality of life can also lead to addiction. It has gotten to the point that some physicians will not even provide narcotic pain medication because they fear they will be unfairly scrutinized and possibly lose their license. leaving pain patients to fend for themselves. And that often means turning to the streets, or to the internet, to get their needed medication.
Those opposed fear that Zohydro will find it’s way into the hand of drug addicts who will very likely abuse the opioid medication. Since there are no additives, the capsules can be broken apart and the contents can be crushed, chewed, snorted, taken with alcohol and even injected.
Those opposed to the FDA approval of Zohydro fear that the United States will face the same problems that they did with another powerful medication, Oxycodone.
So far Attorneys general from at least 28 states have asked the FDA to reconsider their decision.
Interestingly, the FDA approved Zohydro even after its own approval panel voted, 11 to 2, against approval and there are allegations that the drug manufacturer, Alkermes, has close ties with the American Society of Addition Medicine and makes financial contributions to them.
The underlying accusation is that the manufacturers are deliberately putting addictive substances on the market because they know they will reap enormous financial benefits when people find themselves hopelessly addicted and seek treatment.
It truly is a double-edged sword.
Patients that suffer from debilitating and chronic pain should be allowed to find relief for their pain by taking narcotic pain medications such as Zohydro.
Yes, there is the potential for the drug being abused and yes, people will become dependent on the drug, however, those reason should not penalize people who need strong medication to live a quality life.
Medical authorities and the FDA might be better served by focusing their efforts on ensuring that the drugs get into the hands of the patients that need them and not on the street.
Related articles







 Follow
Follow